Main Article Content
Abstract
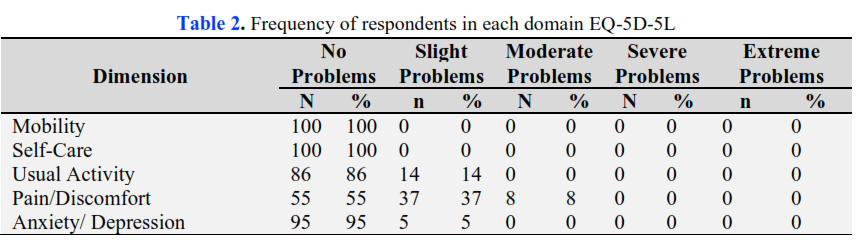
Giving the Sinovac vaccine for Covid 19 can cause several side effects. The existence of side effects that occur, of course, affect their lives, including a decrease in activity, productivity and quality of life. This study aims to determine the HRQoL score in the community after the Sinovac vaccine in Tampan District and Marpoyan District, Pekanbaru City. The research method is analytic observational with a cross-sectional design. The research sample was people who received the first vaccine recorded at the Puskesmas in Tampan District and Marpoyan District, Pekanbaru City, which was selected by purposive sampling technique according to inclusion and exclusion criteria and obtained a sample of 100 respondents. The instrument is a questionnaire EQ5D5L and EQ-VAS. The study results showed that there was little problem with the dimensions of pain (37%) and usual activities (14%), with a score of 0,944 (EQ5D5L) and 0,975 (EQ-VAS). In conclusion, the HRQoL scores on EQ5D5L and EQ-VAS are close to 1, which means close to perfect. This research is expected to be a reference for stakeholders in selecting preventive therapy for Covid 19 and can be used as initial data for cost-utility analysis research.
Keywords
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
- Andayani, T. M. (2013). Farmakoekonomi : prinsip dan metodologi. Bursa Ilmu.
- Chouaid, C., Agulnik, J., Goker, E., Herder, G. J. M., Lester, J. F., Vansteenkiste, J., Finnern, H. W., Lungershausen, J., Eriksson, J., & Kim, K. (2013). Health-related quality of life and utility in patients with advanced non–small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. Journal of Thoracic Oncology, 8(8), 997–1003.
- EuroQol Research Foundation. (2019). EQ-5D-5L User Guide v3.0. version 3. The Netherlands.
- Kementerian Kesehatan Republik Indonesia. (2017). Buku Panduan Penilaian Teknologi Kesehatan. Komite Penilaian Teknologi Kesehatan, Jakarta
- Kementerian Kesehatan Republik Indonesia. (2019). Pedoman Pencegahan dan Pengendalian Coronavirus Disease 2019 (Covid-19). Direktorat Jenderal Pencegahan dan Pengemdalian Penyakit, Jakarta
- KPCPEN. (2021). Peta Sebaran Covid-19. Covid19.Go.Id. https://covid19.go.id/peta-sebaran
- Lidiana, E. H., Mustikasari, H., Pradana, K. A., & Permatasari, A. (2021). Gambaran karakteristik kejadian ikutan pasca vaksinasi covid-19 pada tenaga kesehatan alumni Universitas ‘Aisyiyah Surakarta. Jurnal Ilmiah Kesehatan, 11(1), 11–17.
- Mursyid, A., Haris, R. N. H., Endarti, D., Wiedyaningsih, C., & Kristina, S. A. (2019). Pengukuran Kualitas Hidup Pasien Kanker Payudara di Kota Denpasar menggunakan Instrumen EQ-5D-5L. Jurnal Manajemen Dan Pelayanan Farmasi (Journal of Management and Pharmacy Practice), 9(3), 203–212.
- Purba, F. D., Hunfeld, J. A. M., Iskandarsyah, A., Fitriana, T. S., Sadarjoen, S. S., Ramos-Goñi, J. M., Passchier, J., & Busschbach, J. J. V. (2017). The Indonesian EQ-5D-5L value set. Pharmacoeconomics, 35, 1153–1165.
- Rusmiati. (2021). Pengaruh Komunikasi Tentang Vaksin Sinovac Terhadap Kesadaran Masyarakat Di Desa Jembrak Kabupaten Semarang. MEDFARM: Jurnal Farmasi Dan Kesehatan, 10(1), 18–27.
- Safira, M., Peranginangin, M., & Saputri, G. A. R. (2021). Evaluasi Monitoring Kejadian Ikutan Pasca Imunisasi (KIPI) Vaksin Covid-19 (Coronavac) pada Tenaga Kesehatan di Rumah Sakit Imanuel Bandar Lampung. Jurnal Mandala Pharmacon Indonesia, 7(2), 251–262.
- Sari, M. P., Izzah, A. Z., & Harmen, A. P. (2018). Gambaran Kejadian Ikutan Pasca Imunisasi pada Anak yang Mendapatkan Imunisasi Difteri Pertusis dan Tetanus di Puskesmas Seberang Padang Kota Padang. Jurnal Kesehatan Andalas, 7(3), 352–357.
- Sari, S., Andayani, T. M., Endarti, D., & Widayati, K. (2020). Health-related quality of life in non-small cell lung cancer (NSCLC) patients with mutation of epidermal growth factor receptor (EGFR) in Indonesia. Research Journal of Pharmacy and Technology, 13(1), 443–447.
- WHO. (2021). Side Effect of Vaccines. Who.Int. https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines
- Zhang, Y., Zeng, G., Pan, H., Li, C., Hu, Y., Chu, K., Han, W., Chen, Z., Tang, R., & Yin, W. (2021). Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious Diseases, 21(2), 181–192.
- Zhang, Y., Zeng, G., Pan, H., Li, C., Kan, B., Hu, Y., Mao, H., Xin, Q., Chu, K., & Han, W. (2020). Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. Medrxiv, 2007–2020.