Main Article Content
Abstract
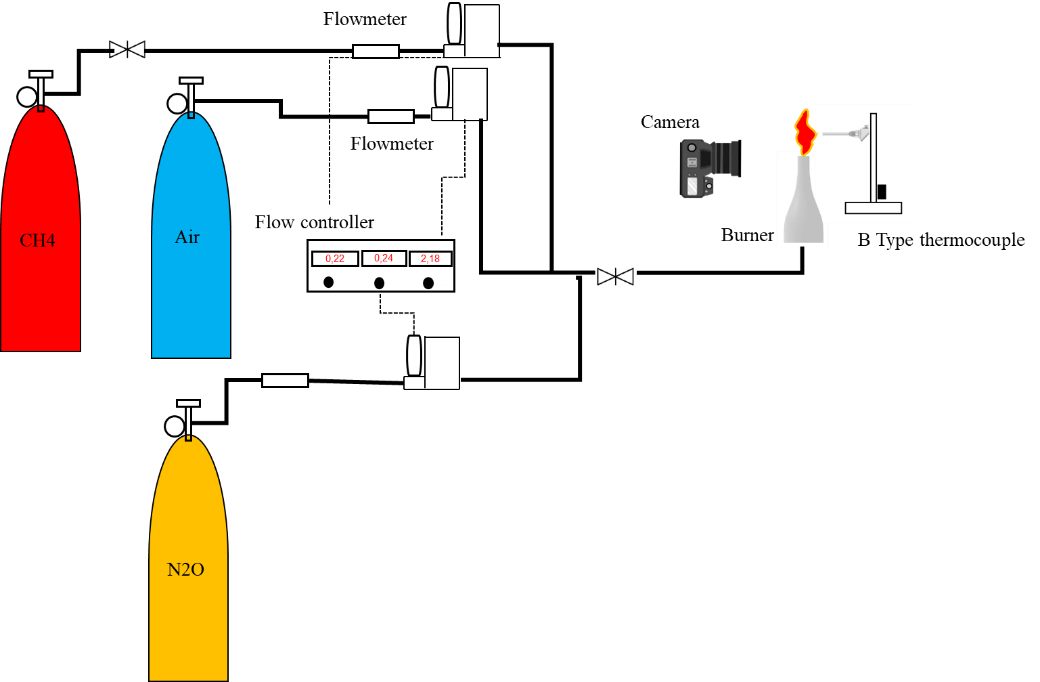
The oxidizer is used in aviation propellants for its relatively high impulse density and non-toxic nature. At elevated temperatures, nitrous oxide (N₂O) decomposes into approximately 33% oxygen (O₂) and 67% nitrogen (N₂), providing a higher oxygen content than ambient air. This decomposition enables N₂O to produce higher flame temperatures than air. Previous studies have shown that N₂O addition improves flame stability in methane combustion systems. This study examined the substitution of O₂ with N₂O in stoichiometric methane–air premixed flames, using both numerical and experimental methods. One-dimensional and two-dimensional simulations with CHEMKIN PRO revealed that replacing air with N₂O increases flame temperature but reduces laminar flame speed, mainly due to lower local oxygen concentrations in the reaction zone. The simulations also showed that nitrogen oxides (NOₓ) emissions increase significantly in the post-reaction zone, while carbon monoxide (CO) and carbon dioxide (CO₂) emissions decrease. Experimental results confirmed that controlled N₂O addition enhances flame stability, but excessive concentrations can trigger combustion instabilities. Overall, the findings indicate that introducing up to 20% N₂O can increase flame temperature and reduce CO emissions in methane flames.
Keywords
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.